Government Chemist case study on Pesticides
The Government Chemist role was created to help protect the public from fraud, malpractice and harm. In 1875, the laboratory of the Government Chemist was appointed as “referee analyst”, a role linked to the Sale of Food and Drugs Act of that year.
The role continues to this day, fulfilling statutory and advisory functions, which are funded by the Department for Business, Energy and Industrial Strategy (BEIS). Part of that function includes resolving disputes in the UK official control system for food and feed, and disseminating the findings from those cases. As mentioned in its 2018 Annual Review, the Government Chemist team saw an interesting case on pesticides.
All foodstuffs intended for human or animal consumption must conform to maximum residue levels (MRLs) for pesticides in order to protect animal and human health. Generally MRLs are recommended by the European Food Safety Authority (EFSA) based on a risk assessment before being adopted into law. Where an MRL has not been specifically set, a “default” MRL of 0.01 mg kg-1 is applied. Products must not be placed on the market as food or feed if they contain a pesticide residue exceeding the prescribed MRL or default level.
Modern pesticide residue analysis by specialist laboratories has progressed to a level where over 350 residues can be detected routinely in several concurrent analytical runs either by gas or liquid chromatography coupled with mass spectrometry.
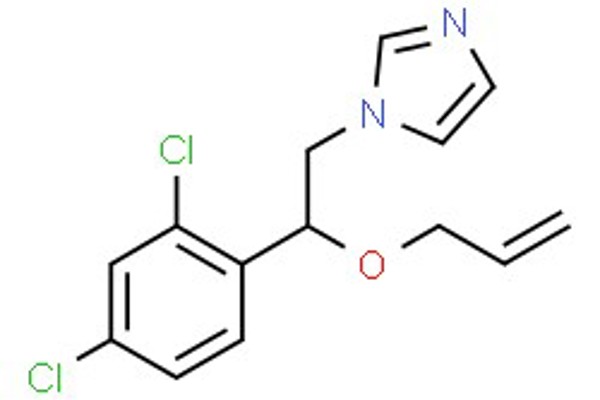
In 2018, a pesticide residue, imazalil (a chlorophenyl substituted imidazole), was reported in an animal feed described as “organic sunflower expeller [cake]”. In fact, sunflower seeds containing imazalil would not be regarded as non-compliant unless the residue concentration was above 0.05 mg kg-1. However, the presence of imazalil in a feed described as “organic” is not acceptable.
Investigation of the milled sample by gas chromatography coupled with mass spectrometry (GC-MS) was shown to provide insufficient analytical certainty.
However, development of an acetonitrile/water QuEChERS extraction and clean-up followed by liquid chromatography coupled with tandem mass spectrometry (LC-MS/MS) was shown to be suitably selective and sensitive for imazalil.
An isotopically-labelled internal standard was applied to yield inherent calculation of recovery. The method of standard additions was applied by fortifying separate aliquots with imazalil standard at the equivalent of nanograms per gram (ng g-1). A reagent blank and instrumental (solvent) blanks, solvent standards and a reference material were included in the analytical batch. The run order was set up to avoid any potential carry over of imazalil. Six mass spectrometric transitions between precursor and product ions were available for imazalil and the analytical batch dataset was examined according to prescribed criteria for identification including retention time window, signal to noise ratio and transition ratio tolerances. Limits of detection (LoD) and quantification (LoQ) of 1.4 ng g-1 and 4.3 ng g-1, respectively, were established from six replicate injections of the referee sample fortified with imazalil at 10 ng g-1 (0.01 mg kg-1). This detailed description of our approach will be of interest to practitioners especially because imazalil was not detected in the sample and the consignment was permitted to enter the supply chain.
In this instance, the consignment owner and analyst requested an opportunity to visit us after the case had been concluded to learn more about the background to the case, details of our analytical approach and to undertake a more general tour of our laboratories.
Read more about the Government Chemist's cases from 2018 here in the latest Annual Review.
