NML collaborate to improve quality assessment schemes for Chronic Myeloid Leukaemia Minimal Residual Disease testing
The National Measurement Laboratory and UK NEQAS for Leucocyte Immunophenotyping announce collaboration to improve quality assessment schemes for Chronic Myeloid Leukaemia Minimal Residual Disease testing.
The National Measurement Laboratory (NML, London, UK) and UK NEQAS for Leucocyte Immunophenotyping (UK NEQAS LI, Sheffield, UK) are pleased to announce a collaborative project aimed at improving the measurement of the BCR::ABL1 fusion gene, the causative genetic mutation in chronic myeloid leukaemia (CML) which occurs during a chromosomal rearrangement leading to formation of the Philadelphia chromosome (Figure 1). Quantification of the BCR::ABL1 fusion gene transcript (messenger RNA) is a critical factor in the management of patients with CML, following treatment with targeted inhibitors of the BCR::ABL1 protein (TKIs), or stem cell transplantation. The collaboration aims to explore the potential of reverse-transcription digital PCR (RT-dPCR) to enhance the accuracy and traceability of BCR::ABL1 quantification in current External Quality assessment (EQA) schemes.
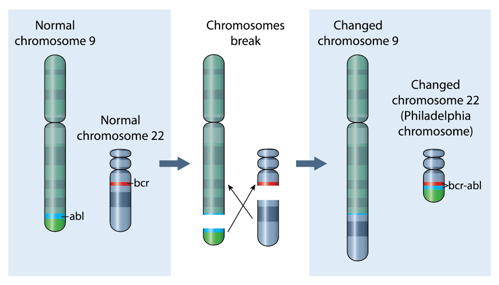
Figure 1: Formation of the BCR::ABL1 fusion gene following reciprocal translocation events between chromosomes 9 and 22.
The project, ‘Feasibility of a Digital PCR-Based Reference Measurement Procedure’, aims to develop an RT-dPCR-based Reference Measurement Procedure (RMP) for BCR::ABL1 Quantification. Clinical analytes are often standardised through a calibration hierarchy involving both Reference Materials and RMPs, which enable traceability of diagnostic assays and ultimately, patient results to higher-order standards. To act as an RMP, superior accuracy is necessary, therefore this project will assess potential technical sources of bias as well as define the precision of BCR::ABL1 measurements. The candidate RMP will be applied to EQA samples from previous rounds to establish comparability with the current consensus-based approach.
The NML hosted at LGC, is an international leader in the measurement science supporting sectors such as health and diagnostics. The Molecular and Cell Biology team within NML has state-of-the-art facilities (Figure 2) and unparalleled expertise in high accuracy quantitative nucleic acid measurements. NML was one of the first laboratories in UK to establish dPCR and is at the forefront of applying the approach to health-related challenges such as cancer genomics, liquid biopsy, infectious disease testing and characterization of advanced therapeutics. "Recent advances in dPCR have been applied in a number of fields to enable standardisation of molecular methods,” says Prof. Jim Huggett, Science Fellow at NML. “This is a timely collaboration that will hopefully underpin the accuracy of CML testing, ultimately leading to better patient outcomes”.

Figure 2: NML Molecular Biology. The laboratory is home to a number of dPCR instruments including the QX200 Droplet Digital PCR System (Bio-Rad) and QIAcuity-4 (QIAGEN) as well as RT-qPCR and sequencing instrumentation.
Liam Whitby, Director at UK NEQAS LI said, "We are extremely excited about this collaboration. By joining forces with NML, we aim to set a new standard in BCR::ABL1 quantification, enhancing the quality of patient care in CML management." With over 15 years of commitment to external quality assessment for BCR::ABL1 Quantification in CML, UK NEQAS LI has been at the forefront of advancements in the field of leukaemia diagnostics. Their BCR::ABL1 Quantification programme, with over 300 participants globally, is specially designed to meet the clinical requirements for CML management and is aligned with the WHO International Scale.
The initial validation performed during the project will provide a foundation for the development of a published, openly available RMP for BCR::ABL1, which has the following potential benefits:
- Facilitate pre-assignment of values to EQA samples, enabling more robust QC prior to initiation of an EQA round
- Allow a harmonised scoring approach for all participants, avoiding potential bias in the consensus values due to their association with the most commonly used molecular methods
- Ensure the EQA programme has the highest levels of metrological traceability to the BCR::ABL1 International Scale, providing direct calibration to the WHO primary standards
- Embed EQA in a metrology framework, ensuring its long-term stability and comparability with other EQA providers
- Availability of the method for value-assignment of other BCR::ABL1 standards produced by commercial providers and academic consortia
- Reduced variation in clinical testing of BCR::ABL1, ensuring the reproducibility of minimal residual disease measurements between laboratories.
Over time, this work will ultimately build on standardisation efforts from the previous two decades and underpin robust clinical decision making in CML patient management.
For more details about the project, please contact Alison Devonshire, Principal Scientist NML alison.devonshire@lgcgroup.com and Stuart Scott, Centre Manager at UK NEQAS LI Stuart.scott@ukneqasli.co.uk
